Wonderful Info About How To Increase Reaction Rate
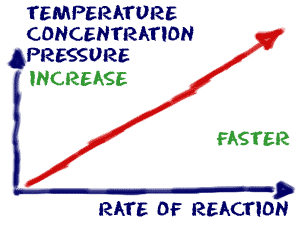
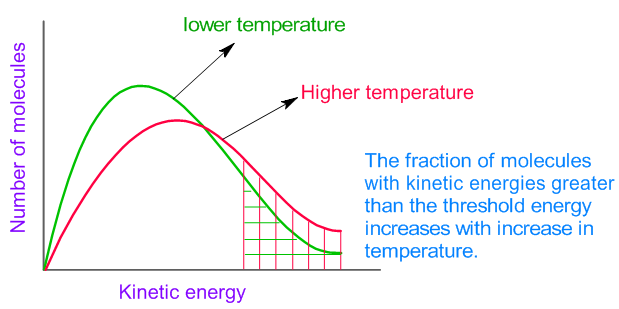
In general, increasing the concentration of a reactant in solution, increasing the surface area of a solid reactant, and increasing the temperature of the reaction system will all increase the rate.
How to increase reaction rate. There are 4 methods by which you can increase the rate of a reaction: Typically, increased concentrations of reactants increases the speed of the reaction,. 17.5 “temperature and reaction rate”).
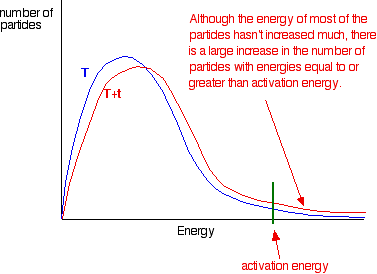
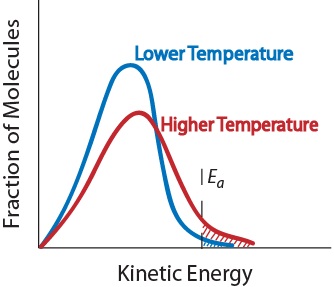
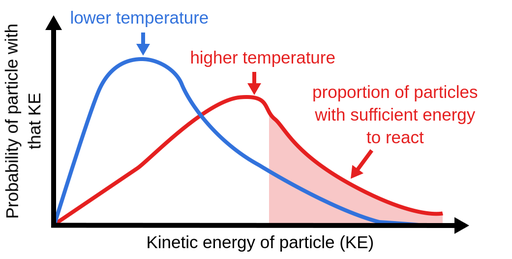
This is because in each case an. More movement = more chances of collision. From the arrhenius equation, it is apparent that temperature is the main factor that affects the rate of a chemical reaction.
This is due to the. Hence, rate of reaction at a given time = gradient of the curve at that instant. In general, increasing the concentration of a reactant in solution, increasing the surface area of a solid reactant, and increasing the.
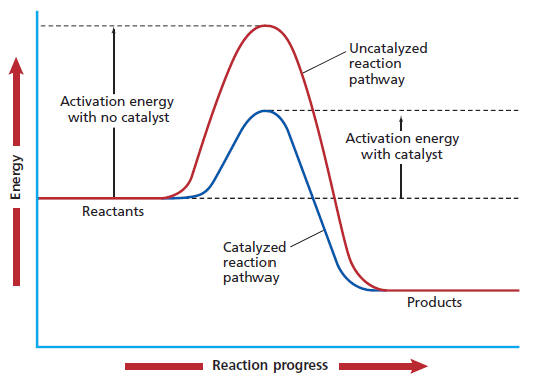
Several factors can increase the rate of a chemical reaction. How do catalysts affect the rate of a reaction? There are 4 methods by which you can increase the rate of a reaction:
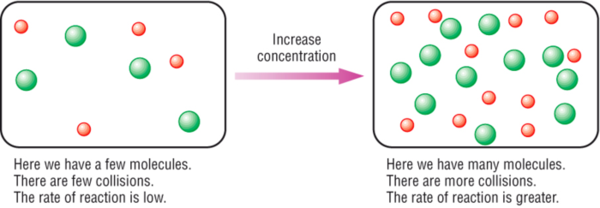
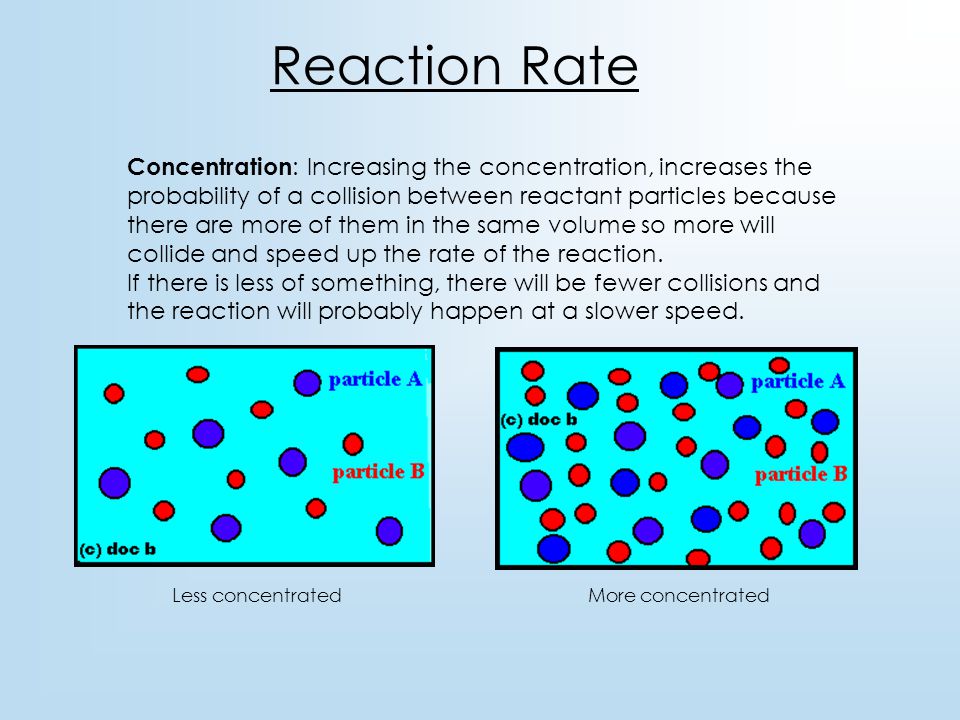
Increase the concentration of a reactant. The rate law uses the molar concentrations of reactants to determine the reaction rate. Reaction rate increases with concentration of reactant.
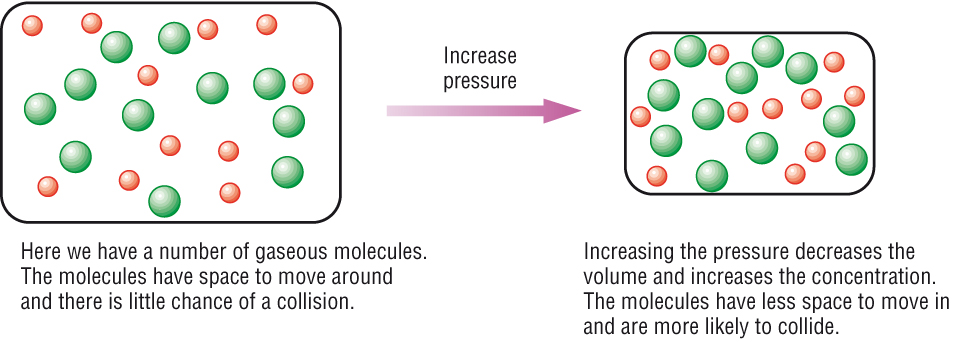
More collisions afford more opportunities for reaction. What are ways to increase the rate of reaction? Concentration (of solutions) surface area, concentration and pressure all have the same effect on reaction rate (an increase leads to a faster reaction rate).